Countries ▼

RHEUMATOID ARTHRITIS
Study design
Amgen offers confidence through robust clinical trial designs
AMGEVITA® has been evaluated in a large, randomised, double-blind, 26-week bioequivalence trial in rheumatoid arthritis, including a treatment switch and long-term open-label extension 1-3

Study population:
Adult patients with moderate-to-severe RA (as defined by the ACR in 2010) for ≥3 months
Active RA: ≥6 swollen joints and ≥6 tender joints
No known history of active tuberculosis
Receiving MTX† with one of the following:
- ESR ≥28 mm/hour
- Serum CRP >1.0 mg/dL and positive for rheumatoid factor or anticyclic citrullinated peptide
Excluded if:
- Received two or more biologic therapies for RA
- Received Humira® or adalimumab biosimilar treatment
Primary endpoint:
Risk ratio (RR) of achieving a 20% improvement from baseline in the American College of Rheumatology (ACR20) core set of measurements at week 24
Key secondary endpoints:
Disease Activity Score 28-joint count-CRP (DAS28-CRP), and the RRs for ACR20, ACR50 and ACR70 response (20%, 50% and 70% improvement in the ACR core set of measurements, respectively) at various time points throughout the trial.
Safety & tolerability assessments
Treatment-emergent adverse events (TEAEs), serious adverse events (SAEs) and incidence of anti-drug antibodies (ADAS)
AEs of interest were also assessed based on the Standard Medical Dictionary for Regulatory Activities (MedDRA) queries
Efficacy results
Comparable efficacy versus Humira®
After the 26-week trial, a comparable proportion of patients met ACR20 (primary endpoint)1‡

A comparable proportion of patients also met ACR50 and 70 response criteria over 24 weeks of treatment1,3

Safety results
Comparable safety, tolerability and immunogenicity versus Humira®
Adverse events were similar between AMGEVITA® and Humira® over the 26-week double-blind period, with no evidence of increased infections. No noticeable differences in safety profile were observed between the 26-week double-blind period and the open-label extension (OLE) of nearly 2 years.1,2
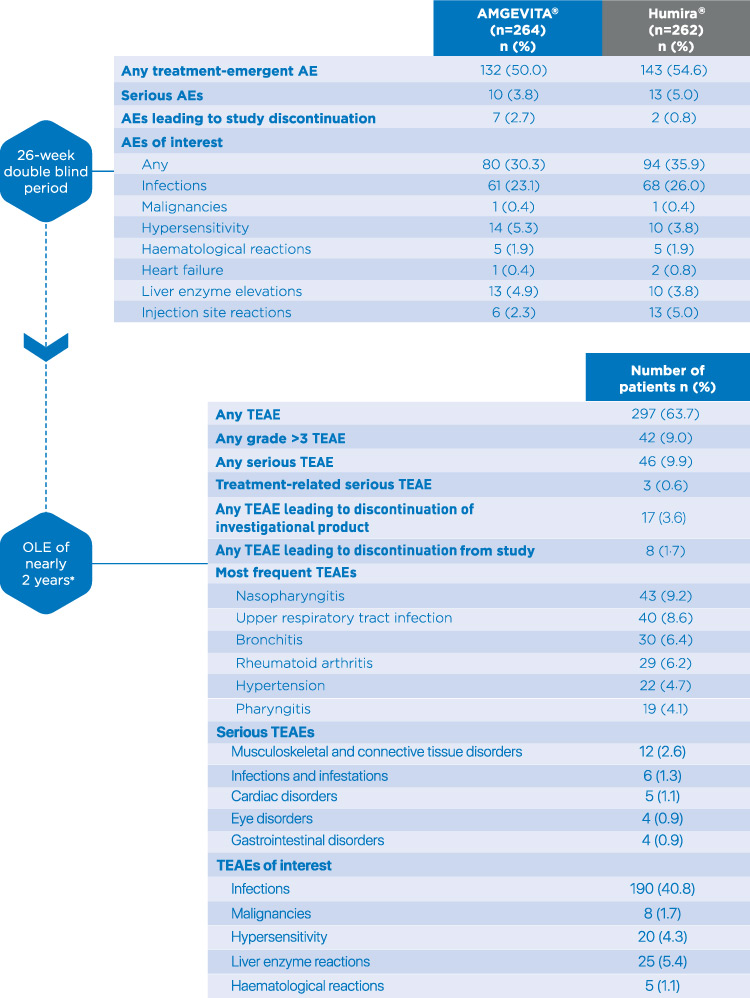
Anti-drug antibody incidence was similar between AMGEVITA® and Humira® up to week 26. The rates of binding and neutralising antibodies in the OLE were similar to those reported during the double-blind period.1,2§

As expected, the incidence rates of binding and neutralising antibodies were increased relative to the parent study baseline and were maintained during the OLE study similarly in the group that transitioned from Humira® to AMGEVITA® and the group that continued on AMGEVITA®.2
ACR: American College of Rheumatology improvement criteria; AE: Adverse event; CI: Confidence interval; CRP: C-reactive protein; E: Total subjects exposure-time (patient-years); ESR: Erythrocyte sedimentation rate; MTX: Methotrexate; OLE: Open-label extension; r: Exposure-adjusted incidence rate per 100 patient-years (100 * n/E); RA: Rheumatoid arthritis; RR: Risk ratio; TEAE: Treatment-emergent adverse event.
*Patients completing the 26-week visit were eligible to continue. Exclusion criteria included the following: experienced a serious AE in the parent study or AE that was considered to be potentially detrimental for long term extension; subject developed an infection requiring the use of intravenous or oral antibiotics; subject completed the parent study but could not be dosed within 4 weeks of the week 26 visit; subject laboratory values for aspartate aminotransferase, haemoglobin, platelet white blood cell, or estimated creatinine clearance during screening for the OLE study; subject had developed a significant concurrent medical condition during the parent study.
†Patients were required to have received MTX for ≥12 consecutive weeks and were on stable oral dose of 7.5–25 mg/week for ≥8 weeks before receiving investigational product.
‡The RR (2-sided 90% CI) of ACR20 at week 24 for AMGEVITA® versus adalimumab was 1.039 (0.954, 1.133) which was well within the predefined equivalence margin (0.738, 1.355), demonstrating clinical equivalence between AMGEVITA® and Humira®.
§Anti-drug antibody status was assessed using a highly sensitive and drug tolerant assay based on the Meso Scale Discovery Electrochemiluminescent platform, followed by a two-tiered test consisting of a screening and specificity assay.
- Cohen S, et al. Ann Rheum Dis. 2017;76:1679–87.
- Cohen S, et al. Arthritis Res Ther. 2019;21:84.
- ABP 501: Background information for the arthritis advisory committee. Submitted to the FDA by Amgen, July 2016.

The information contained in this website is for European healthcare professionals only
I understand and confirm I am an EU healthcare professional
Read more >
Read more >
I am not an EU healthcare professional
Read more >
Read more >
▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.


